Science & Platforms
Our Platform
Exploring the interaction between small molecules and proteins in cellular contexts
We can develop small-molecule drugs against practically any protein of interest because of our rigorous interrogation of the proteome and our proprietary covalent chemistry technologies.
To achieve this, we built a platform that combines a unique interdisciplinary suite of technologies to accelerate the discovery and development of precision medicines. It integrates chemoproteomics, AI and direct-to-biology approaches to map covalent binding sites, identify actionable ligands, and enable accelerated optimization to a clinical candidate.
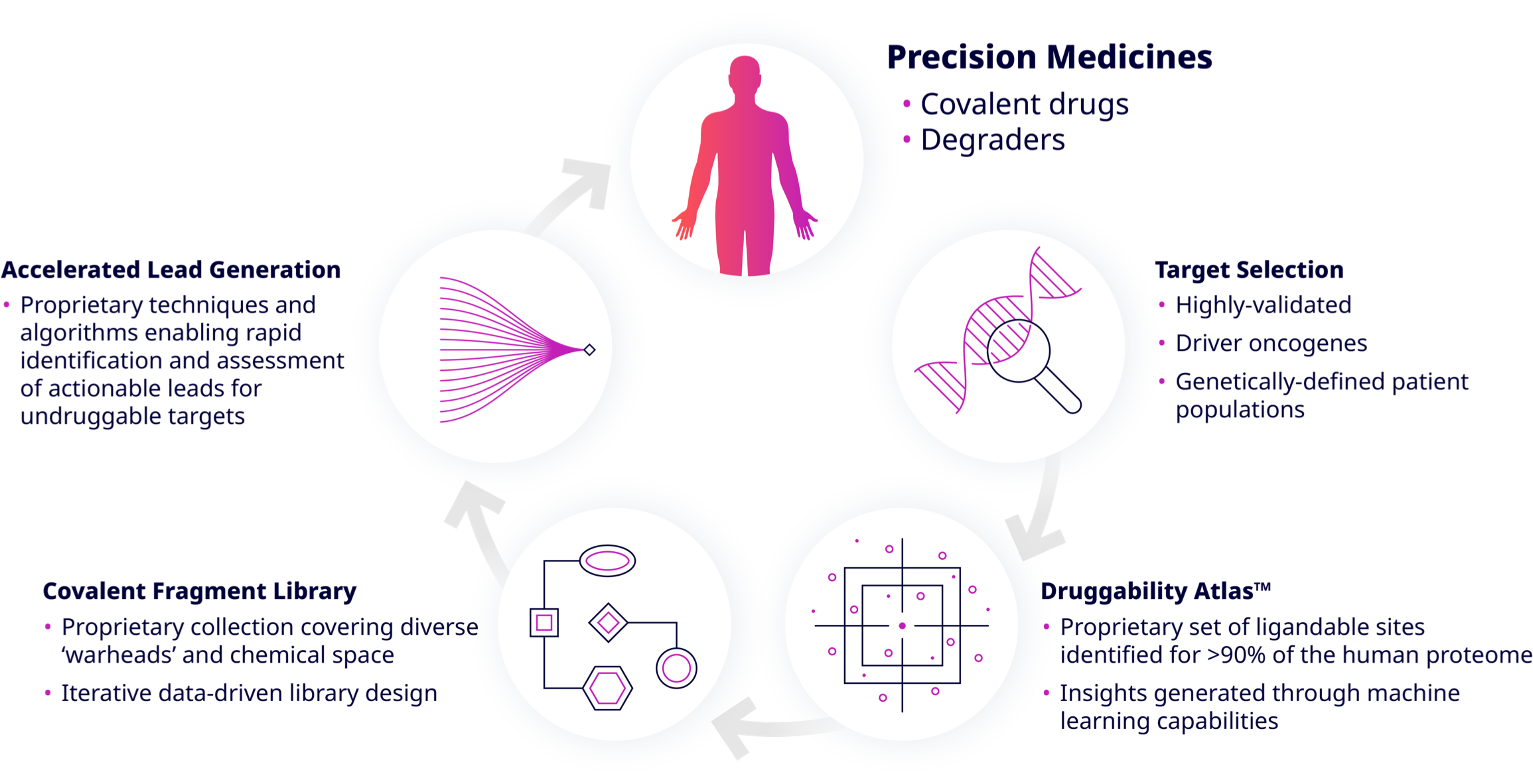
Understanding Frontier’s Technology
Druggability AtlasTM
The Druggability AtlasTM merges our own proteomic data with the scientific literature on disease-driving targets and allows large-scale data analysis to provide a guidebook for drugging nearly any protein in the human proteome. It identifies actionable disease targets in all pathways and cell compartments, and it has uncovered and characterized more than 150,000 covalent binding sites across nearly the entire human proteome and actionable ligands for more than 8,000 targets.
Covalent Fragment Library
We have generated an AI-optimized, industry-leading covalent fragment library. This library features different tunable warhead classes and is capable of targeting multiple residue types on any protein of interest. Our library goes well beyond industry gold standards and greatly expands access to productive covalent chemical space.
Accelerated Lead Generation
We are accelerating covalent drug discovery in an unprecedented manner. Using conventional methods, it takes 1 – 1.5 years to discover chemical matter, which, in the end, may not be actionable. In total, it takes 3 – 5 years to find a clinical candidate.
Our Druggability Atlas enables us to start on day one with actionable chemical matter. Ligand optimization is accelerated with direct-to-biology, high-throughput chemistry technology as well as AI-driven covalent drug design, so that we can advance to a clinical candidate in 1.5 – 2 years.
Artificial Intelligence
AI optimizes everything we do. Leveraging our vast proprietary chemoproteomic data (>2 billion data points) together with LLM/PLM-powered structural insights, we enrich the Druggability AtlasTM and prioritize targets, binding sites, and ligands. We also use AI to build our covalent fragment library, enabling us to optimize the reactivity range of compounds and focus on actionable chemistry. AI accelerates our ligand optimization process via tailored covalent chemistry property models and an autonomous covalent drug design engine.
Artificial intelligence approaches are also applied to generate and curate Frontier’s covalent fragment library.
Frontier has developed and deployed algorithms that determine the desired chemical property space and reactivity profiles based on the latest data. Such data-driven insights enable us to focus on the most promising areas of chemical space.
These machine learning applications enable Frontier to generate an expanding library of high quality and diverse compounds against key targets and their prioritized hotspots. This opens novel opportunities to enable the development of innovative precision-based medicines.
Covalent AI™ is enabled by Frontier’s yearslong generation of large diverse covalent data. The Covalent Ground Truth (CoGT™) is the basis of all Covalent AI algorithms